News and Publications
Press Releases
April 10, 2025
Evecxia Therapeutics Announces Appointment of C.R. Sincock II to the Board of Directors
October 25, 2024
September 18, 2024
Evecxia Therapeutics ACCESS CHINA Partnering Forum
July 23, 2024
Evecxia Therapeutics Files Patent for 5-hydroxytryptophan (5-HTP) Chemical Synthesis
October 30, 2023
October 2, 2023
Evecxia Therapeutics Reports No Toxicology Findings in Nonclinical GLP Studies of Adjunctive EVX-101
September 14, 2023
Evecxia Therapeutics Announces CEO Transition
May 25, 2023
April 28, 2023
April 10, 2023
March 15, 2023
June 13, 2022
Evecxia Therapeutics Announces Leadership Transition to Support Next Phase of Development and Growth
May 24, 2022
October 28, 2020
Evecxia Therapeutics Appoints Thomas Aasen to Board of Directors
June 30, 2020
December 5, 2019
Evecxia Inc. Appoints Jacob Jacobsen, PhD, as Chief Scientific Officer
May 13, 2019
May 8, 2019
October 11, 2018
Evecxia licenses additional IP from NUS, NUH and NTU in Singapore
August 28, 2018
Evecxia licenses additional IP from Duke University
August 16, 2018
Publications
VIDEO: Fireside chat with Professor George Papakostas, Professor of Psychiatry, Harvard Medical School.
Depression responding inadequately to first-line antidepressants and the promise of adjunctive EVX-101 to address this critical unmet need.
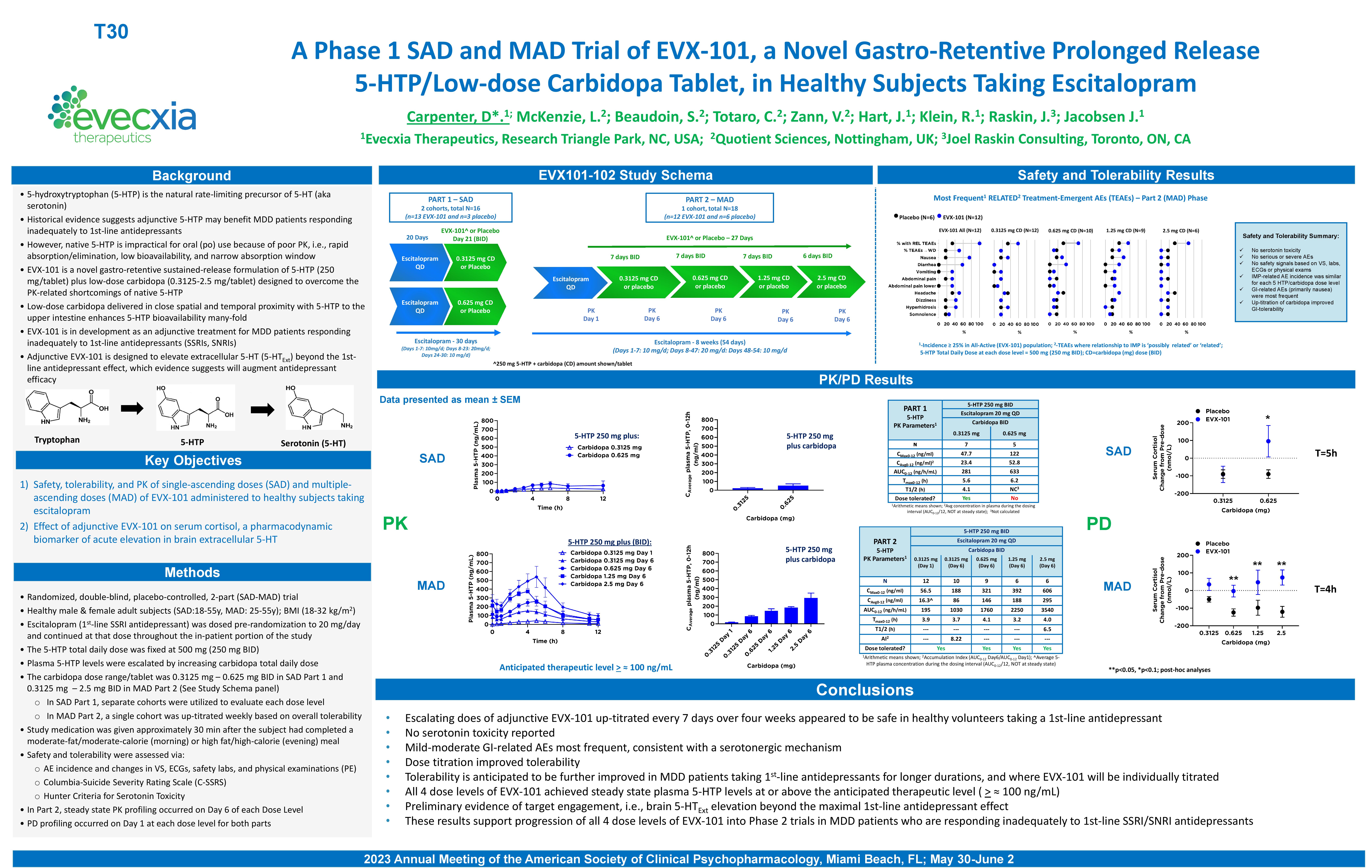
A Phase 1 SAD and MAD trial of EVX-101, a novel gastro-retentive prolonged release 5-HTP/low-dose carbidopa tablet, in healthy subjects taking escitalopram.
Carpenter, D., McKenzie, L., Beaudoin, S., et al. A Phase 1 SAD and MAD trial of EVX-101, a novel gastro-retentive prolonged release 5-HTP/low-dose carbidopa tablet, in healthy subjects taking escitalopram. American Society of Clinical Psychopharmacology (ASCP) Annual Meeting, May 30-June2, 2023.
This Phase 1 double-blind, placebo-controlled, single-ascending dose (SAD) and multiple-ascending dose (MAD) trial demonstrated that escalating doses of EVX-101 up-titrated every 7 days over 4 weeks appeared to be safe in healthy volunteers taking a first-line anti-depressant. No serious or severe adverse events were reported. Mild-moderate gastrointestinal-related AEs were most frequently reported, consistent with a serotonergic mechanism. Tolerability was improved by titration. At steady state, all administered dose levels achieved 5-HTP plasma levels at or above the targeted therapeutic level (≥100 ng/ml). Evidence of target engagement was also demonstrated, i.e., elevation of brain extracellular serotonin beyond the first-line antidepressant effect.
Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept
Jacobsen JPR, Oh A, Bangle R, et al. Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept. Neuropsychopharmacology. 2019;44(12):2082-2090.
This comprehensive study was designed to optimize translational fidelity, It reports that high-dose oral adjunctive 5-HTP slow-release in mice is safe and effective in amplifying brain serotonin neurotransmission beyond the SSRI effect, as assessed by behavioral, biochemical, and gene transcription measures.
Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: clinical and preclinical rationale
Jacobsen JPR, Krystal AD, Krishnan KRR, et al. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: clinical and preclinical rationale. Trends Pharmacol Sci. 2016;37(11):933-944.
This paper reviews the multipronged clinical data supporting that adjunctive 5-HTP SR could treat depression patients responding inadequately to first-line SSRI/SNRI antidepressants. That clinical data relates to earlier 5-HTP clinical research as well as clinical research with serotonergic antidepressants and compounds more broadly. Key supportive preclinical data is reviewed as well.
SSRI augmentation by 5-hydroxytryptophan slow release: mouse pharmacodynamic proof of concept
Jacobsen JP, Rudder ML, Roberts W, et al. SSRI augmentation by 5-hydroxytryptophan slow release: mouse pharmacodynamic proof of concept. Neuropsychopharmacology. 2016;41(9):2324-2334.
This study reports that 5-HTP slow-release synergizes with chronic SSRI in elevating extracellular serotonin in mice. It further demonstrates, assessing across a variety of physiological and behavioral measures, that slow-release delivery dramatically improves the safety and tolerability of 5-HTP administration.