Pipeline
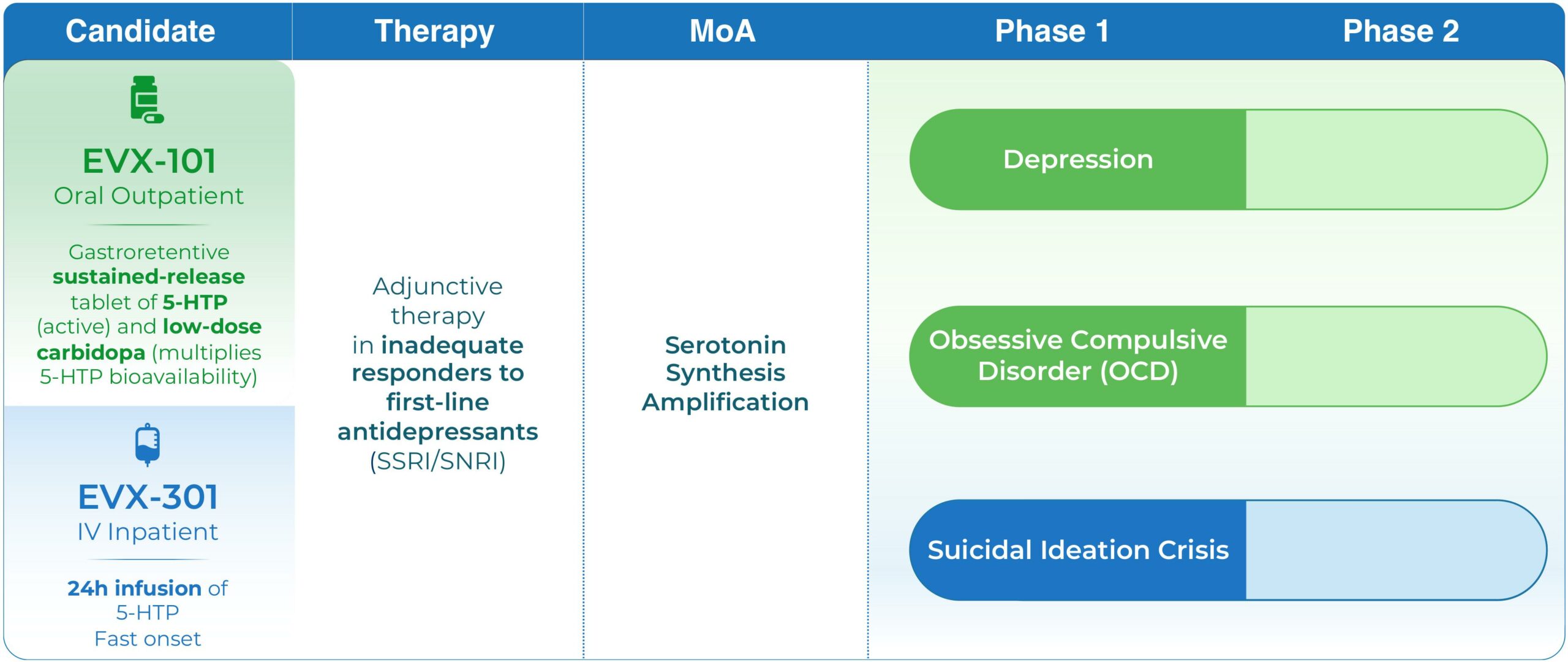
EVX-101
Evecxia Therapeutics is developing EVX-101 as an adjunctive (add-on augmentation) therapy for depression patients responding inadequately to first-line SSRI/SNRI antidepressants monotherapy.
The adjunctive EVX-101 therapeutic rationale in depression is based in clinical evidence (see below). This represents a critical risk-mitigation aspect. All marketed and late development stage antidepressants are based in clinical evidence—e.g., serendipity, pilot trials, pathology—from iproniazid in the 1950s, to atypical antipsychotics and ketamine in the 2000s, to navacaprant in the 2020s. (Preclinical behavioural, neuroplasticity, and cell culture models have yet to show their mettle).
Risk-mitigated proprietary pharmaceutical approach
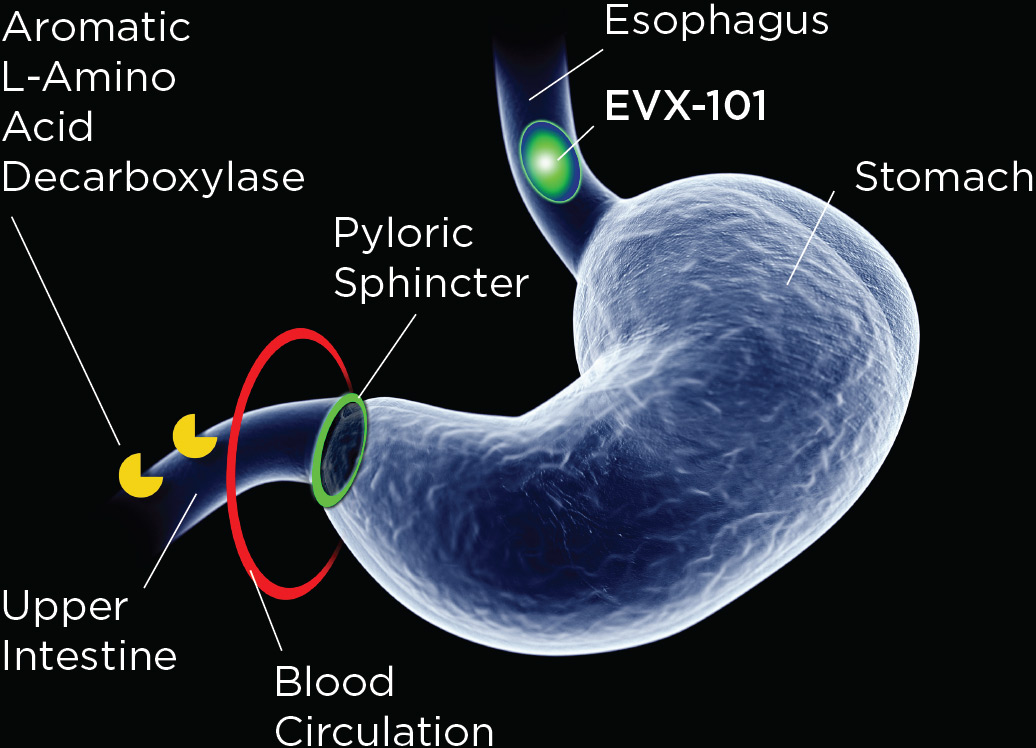
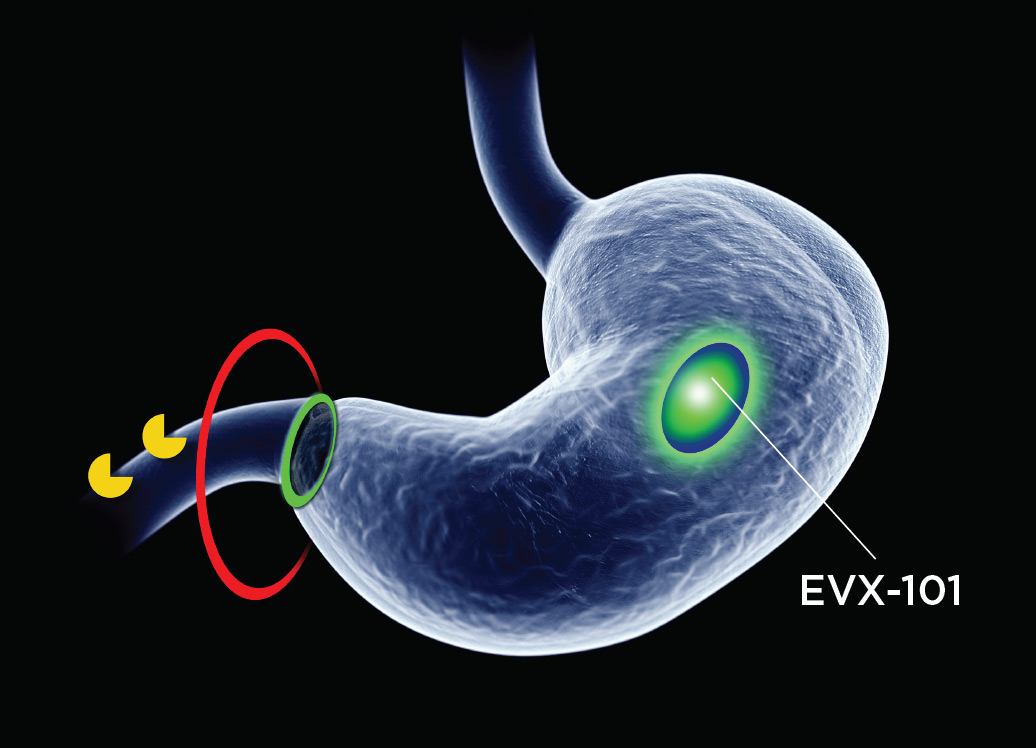
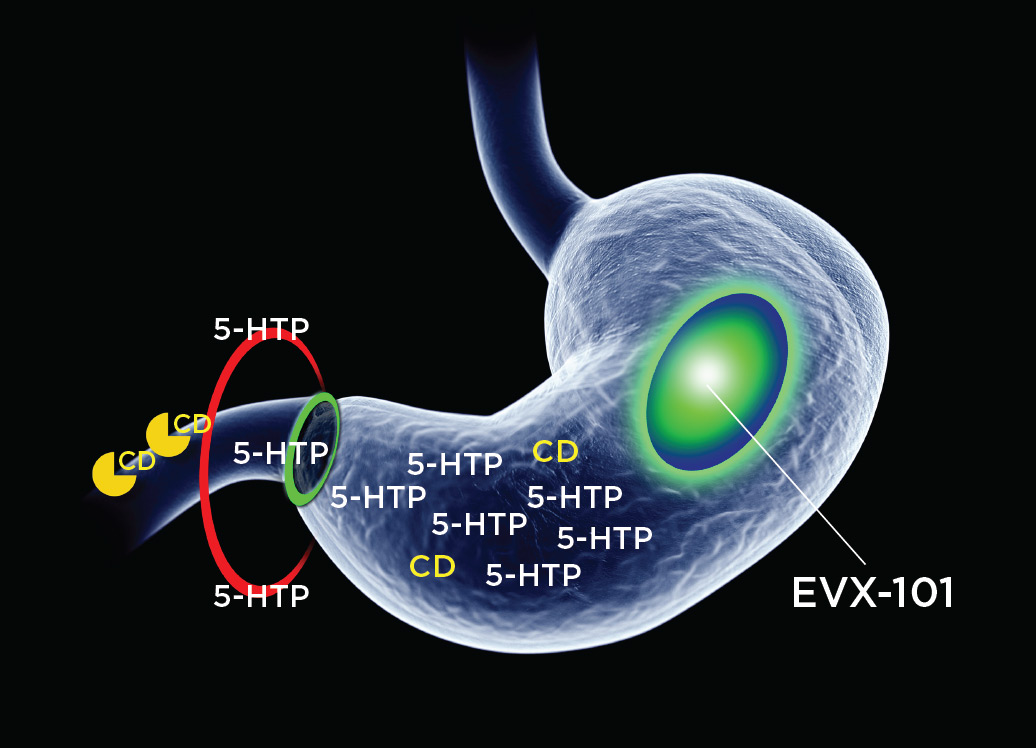
EVX-101 is a proprietary, oral tablet, gastroretentive, slow-release formulation of 5-HTP and low-dose carbidopa. EVX-101 uses a novel embodiment of a validated gastroretentive drug delivery technology, used in 8 FDA-approved drug products. The gastroretentive technology ensures sustained delivery of 5-HTP and carbidopa over about 8h to the site of 5-HTP absorption, the upper intestine. The low-dose carbidopa protects 5-HTP against first-pass metabolism (to serotonin) over the intestinal wall, thereby manyfold increasing the oral systemic bioavailability of 5-HTP. Thus, EVX-101 ensures sufficient and sustained 5-HTP plasma exposure, enabling sustained brain serotonin synthesis amplification and realizing the putative therapeutic potential across depression and other CNS disorders.
EVX-101 can be taken by the patient at home, with breakfast and dinner. EVX-101 is expected to be free of abuse potential and therefore not considered a controlled substance by the US Drug Enforcement Agency. EVX-101 is expected not to cause dissociation or impairment and limiting driving or social and vocational functioning.
EVX-101, adjunctive or as monotherapy, could have therapeutic relevance in additional important unmet needs in Psychiatry e.g. OCD, and in other disorders of the brain.
EVX-101 is protected by a portfolio of issued and pending patents, in the US, EU, and major overseas markets.
Risk-mitigated therapeutic rationale of adjunctive EVX-101 in depression
When added to existing SSRI/SNRI first-line antidepressant therapy, adjunctive EVX-101 will amplify serotonin neurotransmission beyond the antidepressant effect—specifically elevate extracellular serotonin beyond the effect of SSRI/SNRI first-line antidepressant monotherapy—which clinical data suggest will augment the antidepressant response in patients inadequately treated by first-line SSRI/SNRI antidepressant monotherapy.
Specifically:
- First-line antidepressants elevate frontal cortex extracellular (active) serotonin only modestly, inconsistently, and with a delay, as determined by in vivo brain PET imaging - and treat depression only modestly, inconsistently, and with a delay.
- These pharmacological and therapeutic limitations are unsurprising, as the molecular target, the serotonin transporter, has very low expression in the human cortex, as determined by post-mortem autoradiography and in vivo PET imaging.
- Since the 1970s (i) multiple clinical pilot studies and (ii) experimental clinical practice using a variety of compounds administered adjunctively to an SSRI or another serotonin reuptake inhibitor, have aimed to treat depression responding inadequately to first-line antidepressant effect, by elevating extracellular serotonin beyond the effect of first-line antidepressant monotherapy. The compounds in question, administered adjunctively in clinical trials or in practice, include 5-HTP (immediate serotonin precursor), tryptophan (a few percent become 5-HTP, then serotonin), methylfolate (co-factor in serotonin synthesis), monoamine oxidase inhibitors (inhibits metabolism of serotonin and other monoamines), and thyroid hormones (believed to act via further elevating extracellular serotonin). The resultant antidepressant augmentation findings are generally positive - despite the absence, in most cases, of validated doses, validated pharmacokinetics, appropriate dosage forms, and adequately powered trials.
- Adjunctive 5-HTP has been reported in humans and animals to potently elevate extracellular serotonin beyond SSRI effect, in a synergistic fashion, and with a wide dynamic range.
Thus, elevation of brain extracellular serotonin / enhancing serotonin neurotransmission beyond the effect of first-line SSRI/SNRI antidepressant monotherapy represents a clinically risk-mitigated antidepressant augmentation approach for depression when first-line SSRI/SNRI antidepressant monotherapy is inadequately. EVX-101 would be the first FDA-approved drug product to leverage this risk-mitigated antidepressant augmentation pharmacology.
EVX-101 Phase 1 Trials
Evecxia has successfully completed two Phase 1 clinical trials of EVX-101
A Phase 1a trial was an open-label trial in healthy volunteers to evaluate the 5-HTP bioavailability and PK profile following EVX-101 administration. The trial aim was to optimize 5-HTP/carbidopa doses and the EVX-101 formulation composition. Adverse events were mild or moderate with no safety signals. There were no serious adverse events.
A Phase 1 trial was a double-blind, placebo-controlled, single ascending dose (SAD), multiple ascending dose (MAD) titration trial in healthy subjects receiving an SSRI (escitalopram) at steady state to evaluate safety, tolerability, 5-HTP pharmacokinetics, and neuroendocrine target engagement when EVX-101 was administered adjunctively to the SSRI. Four carbidopa dose levels (0.3125 mg to 2.5 mg per tablet) with fixed dose 5-HTP (250 mg per tablet) were evaluated. All four carbidopa dose levels produced 5-HTP exposure in or above the anticipated therapeutic range, estimated at CAverage ~100 ng/ml. Carbidopa enhanced 5-HTP bioavailability up to 10-fold, with a clear dose-response relationship. Carbidopa plasma levels were mostly below detection. Thus, low-dose carbidopa appears to act selectively in the upper intestine, protecting 5-HTP against first-pass metabolism in the upper intestine, thereby effectively enhancing 5-HTP’s bioavailability, and without the carbidopa affecting other peripheral organs. Neuroendocrine biomarker data suggested robust target engagement, i.e., elevation of brain extracellular serotonin beyond the SSRI effect, across several carbidopa dose levels. Adverse events were mild or moderate. There were no safety signals of concern. There were no serious adverse events.
These Phase 1 data strongly support EVX-101 as an adjunctive antidepressant candidate. A Phase 2 study of adjunctive EVX-101 in depression patients with inadequate response to first-line SSRI/SNRI antidepressants is expected to commence enrolment late 2024.
EVX-101 combines gastroretention with low-dose carbidopa to druggify 5-HTP
EVX-301
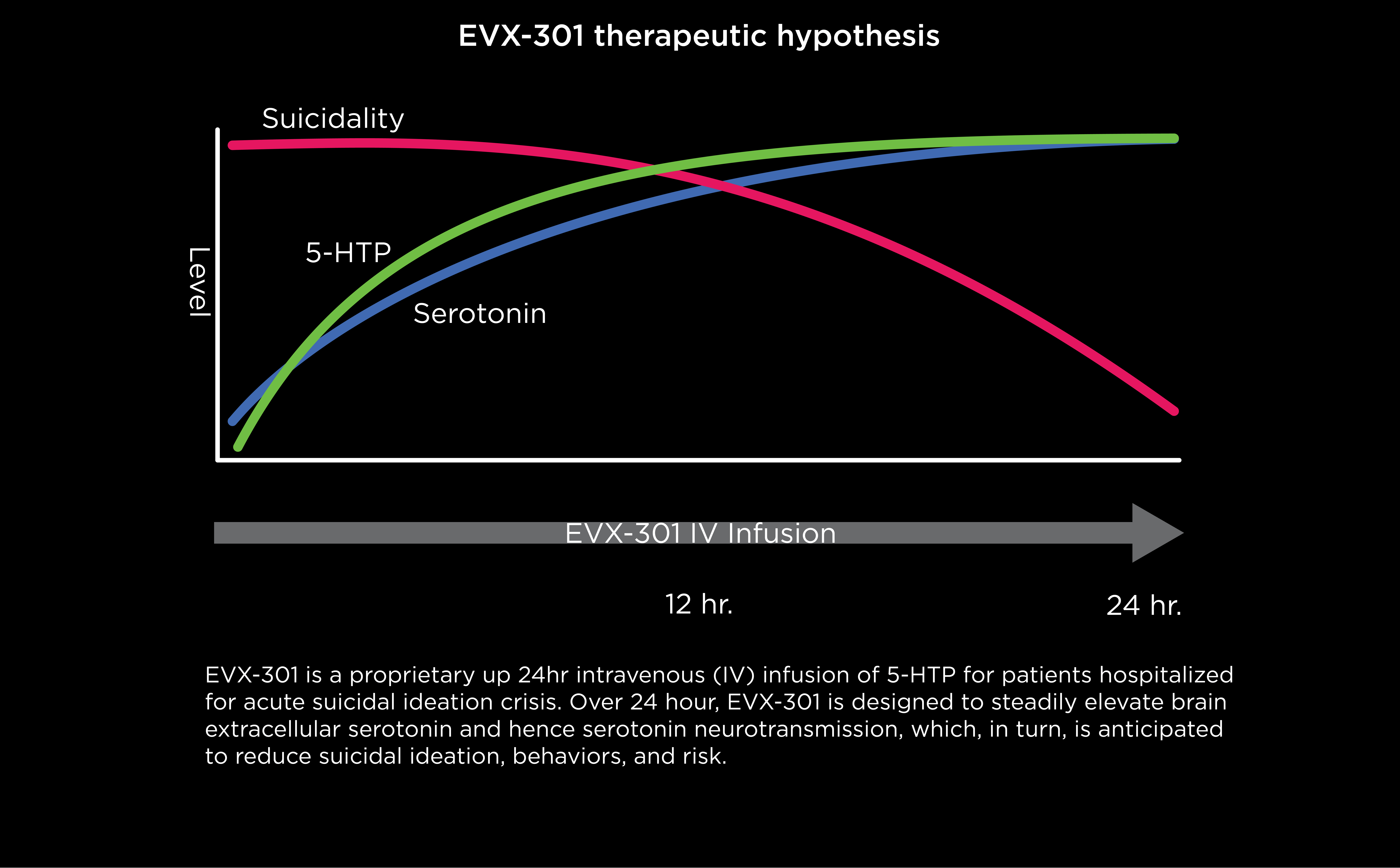
Evecxia Therapeutics is developing EVX-301 as a rescue therapy for patients hospitalized for acute suicidal ideation crisis. EVX-301 is a proprietary intravenous up to 24h infusion of 5-HTP.
Risk-mitigated therapeutic rationale of adjunctive EVX-301 in suicidal ideation
EVX-301 is designed to quickly and optimally amplify brain extracellular serotonin and hence serotonin neurotransmission. Convergent clinical data suggest this will impart an anti-suicidal effect, comprised of improved mood, sociability, and calmness, and reduced aggression and impulsivity.
Specifically:
- Meta-analyses find long-term first-line SSRI antidepressant therapy to protect against suicidal ideation, albeit modestly, consistent with the modest elevation of extracellular serotonin after long-term first line antidepressant therapy. (Short-term first-line antidepressant temporarily decrease cortex extracellular serotonin, coinciding with potentially increased suicidal ideation).
- The suicidal ideation affective archetype is characterized by (i) aggression, (ii) impulsivity, (iii) low mood, and (iv) feeling socially disconnected. In humans, acutely elevating extracellular serotonin—e.g., with fenfluramine, tryptophan, or 5-HTP—has been reported to decrease (i) aggression and (ii) impulsivity and (iii) improve mood and (iv) social connectedness.
Thus, acute extracellular serotonin elevation represents a putative anti-suicidal pharmacology, which EVX-301 is optimized to achieve, with fast onset.
EVX-301 therapeutic use
The patient will receive EVX-301 in the clinic or hospital. Evecxia expects that EVX-301 can be a stand-alone rescue therapy in its own right, to stabilize the patient. Additionally, EVX-301 could be the first step in a novel treatment algorithm, the second step being transfer to long-term suicide ideation prevention therapy with EVX-101. Thus, EVX-301 alone or with EVX-101 could become critical tools in combatting the US crisis in suicide and suicidal ideation.
EVX-301 Phase 1 Study
Evecxia has successfully completed a Phase 1 clinical trial of EVX-301
This Phase 1 trial was a double-blind, placebo-controlled, single ascending dose (SAD) trial in healthy subjects receiving an SSRI (escitalopram) at steady state to evaluate safety, tolerability, 5-HTP pharmacokinetics, and neuroendocrine target engagement when EVX-301 was administered adjunctively to an SSRI.
EVX-301 dose-dependently increased 5-HTP plasma exposure with minimal variation between subjects. Importantly, adjunctive EVX-301 could achieve clinical target 5-HTP plasma exposure, CAverage ~100 ng/ml, within hours, with no adverse events, and with neuroendocrine biomarker data suggesting robust target engagement, i.e., elevation of brain extracellular serotonin beyond the SSRI effect. Adverse events at higher doses were mild or moderate, with minimal safety signals detected.
These Phase 1 data support EVX-301 as a putative rescue therapy for acute suicidal ideation crisis. A Phase 2a trial of adjunctive EVX-301 in depression patients with inadequate response to first-line antidepressants and ongoing suicidal ideation is expected to commence enrolment in Q3/2024.
Early Phase Programs
 Serotonin synthesis amplification may have broad therapeutic potential. For instance, in clinical studies, 5-HTP administration has shown direct evidence of therapeutic efficacy across a spectrum of psychiatric, neurological, endocrinological, and developmental disorders. Further, there are rationales for that serotonin synthesis amplification could treat yet other disorders.
Serotonin synthesis amplification may have broad therapeutic potential. For instance, in clinical studies, 5-HTP administration has shown direct evidence of therapeutic efficacy across a spectrum of psychiatric, neurological, endocrinological, and developmental disorders. Further, there are rationales for that serotonin synthesis amplification could treat yet other disorders.
Evecxia is actively exploring additional indications for EVX-101 and EVX-301 and additional drug candidates utilizing 5-HTP as the active compound.