Pharmaceutical Approach
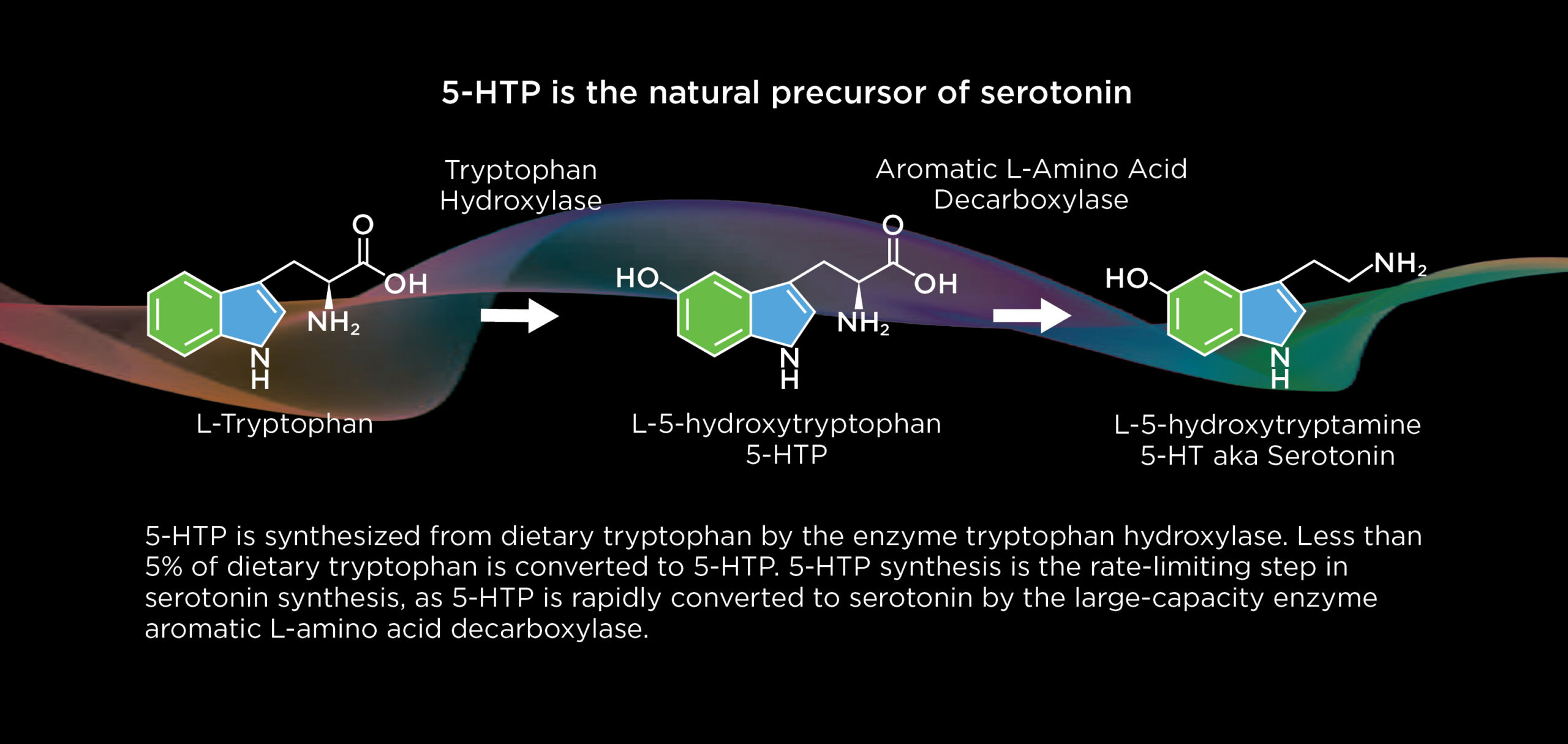
Brain serotonin synthesis amplification
Evecxia seeks to leverage the therapeutic potential of serotonin synthesis amplification - building a stronger, more dynamic, and more resilient endogenous brain serotonin system. Serotonin synthesis amplification is a unique mode of serotonin system modulation, distinct from targeting serotonin transporters (e.g., SSRIs/SNRIs) or receptors (e.g., psychedelics). Serotonin synthesis amplification is not utilized by any FDA/EMA-approved drugs; yet, convergent clinical trial evidence from the literature points to broad therapeutic potential of serotonin synthesis amplification across psychiatric and neurological disorders. Evecxia leverages 5-hydroxytryptophan (5-HTP), delivered via proprietary pharmaceutical technologies, to for the first time enable the therapeutic potential of serotonin synthesis amplification.
Exogenous 5-HTP And Brain Serotonin
5-HTP can also be administered directly to the body. Numerous human and animal studies demonstrate that 5-HTP administered to the body, orally or intravenously, reaches the brain and is converted to serotonin by serotonergic neurons, thereby amplifying serotonin synthesis. At sufficient levels, exogenous 5-HTP will elevate extracellular serotonin and hence enhance serotonin neurotransmission, as demonstrated by multiple biomarker and brain imaging studies. Thus, exogenously administered 5-HTP can amplify brain serotonin levels and strengthen serotonin neurotransmission.
5-HTP Human Safety
5-HTP has a remarkably good human safety record. 5-HTP has been administered to thousands of human subjects in hundreds of studies since the 1950s. Many studies administered high (>1 gram/day) 5-HTP doses, often for long durations (months, years), and often with other drugs, including SSRI or SSRI-like drugs. 5-HTP has not been found to cause any severe side effects, human disease, or deaths.
Recently, Evecxia completed a Phase 1 SAD/MAD trial for EVX-101 and a Phase 1 SAD trial for EVX-301, both drug candidates administered to healthy volunteers concurrently treated with a first-line antidepressant (escitalopram, an SSRI). Both trials reported excellent safety profiles.
5-HTP Therapy For Human Diseases – Promise & Limitations
In small clinical trials, involving thousands of patients, and in experimental clinical practice 5-HTP has shown promising therapeutic potential in a wide spectrum of human disorders, most notably depression, but also in obesity, myoclonus, movement disorders, pain disorders, insomnia, and some rare childhood disorders. Despite these promising therapeutic findings, no 5-HTP drug candidate has ever entered regulatory-grade clinical trials. No FDA- or EMA-approved 5-HTP drug product exists. Likely, this is because the native 5-HTP molecule is highly impractical as a drug therapy, due to 5-HTP’s (i) poor oral bioavailability, (ii) poor pharmacokinetics, and (iii) narrow absorption window restricted to the upper intestine.
Native 5-HTP, when taken orally, is mostly converted to serotonin in the intestine. Hence, normally only a fraction—less than 10 %—of orally ingested 5-HTP reaches the blood stream and the brain. While 5-HTP appears to have good safety, the tolerability of native, immediate release, 5-HTP is often sub-optimal. What 5-HTP is absorbed from the intestine is absorbed rapidly. This can cause transient 5-HTP spikes and hence serotonin spikes and bothersome adverse events, manifest usually as nausea and other gastrointestinal events. Such CMax-effects are common for fast-absorbed compounds. Conversely, as native 5-HTP has a short half-life—2-3h in humans—5-HTP plasma levels drop off between administrations, necessitating three or more daily doses to maintain a just reasonably sustained 5-HTP plasma levels and serotonin amplifying effect. Moreover, Evecxia discovered that 5-HTP has a narrow absorption window in humans, restricted to the upper intestine, with minimal 5-HTP absorption occurring in the colon.
A novel drug delivery technology that overcame the triple shortcomings of 5-HTP as a drug therapy could, for the first time, realize the full therapeutic potential of 5-HTP and serotonin synthesis amplification - in depression, and in other mental and central nervous system disorders. EVX-101 is the first such drug candidate.
Therapeutic Rationale
Sustained Amplification Of Brain Endogenous Serotonin By EVX-101
Oral dosing with native 5-HTP is associated with limited absorption, intermittent exposure, and suboptimal tolerability. These shortcomings make native 5-HTP a poor drug. Evexcia’s pharmaceutical hypothesis posits that if 5-HTP plasma spikes and valleys are minimized, and 5-HTP's absorption enhanced, the ability of 5-HTP to amplify brain serotonin synthesis will be transformatively improved, enabling the full therapeutic potential to be realized. Research at Duke University by Evecxia’s founders Drs. Jacobsen and Caron confirmed this hypothesis in translational pre-clinical pharmacokinetic, pharmacodynamic, and toxicology models.
Mechanism Of Action Of EVX-101 Vs First-Line Antidepressants
First-line antidepressant are the selective serotonin reuptake inhibitors (SSRIs) and the serotonin norepinephrine reuptake inhibitors (SNRIs). First-line antidepressants treat depression, as well as anxiety disorders, by elevating extracellular serotonin in the frontal cortex, which activates post-synaptic serotonin receptors, enhances serotonin neurotransmission in the frontal cortex, and causes the antidepressant effect. First-line antidepressants work predominantly by blocking the serotonin reuptake transporter, one of several regulators of extracellular serotonin. However, human frontal cortex levels of serotonin transporters are low. In human brain imaging studies SSRIs elevate extracellular serotonin modesty and inconsistently across subjects, and with a delay. The first-line antidepressants’ inherently limited pharmacology may contribute to the generally modest, inconsistent, and delayed therapeutic effect of first-line antidepressants.
In contrast, EVX-101 amplifies endogenous serotonin synthesis and levels, independently of the serotonin transporter, thereby amplifying dynamic serotonin neurotransmission.
Adjunctive EVX-101 Synergizes With First-Line Antidepressants To Enhance The Serotonergic Therapeutic Mechanism
"Adjunctive" means adding-on a second treatment to an already ongoing treatment to augment clinical efficacy. Adjunctive drug therapy is common in Psychiatry. Evecxia’s pre-clinical research demonstrated that adjunctive 5-HTP sustained-release (5-HTP SR, modeling EVX-101) interacts synergistically with ongoing SSRI treatment to elevate extracellular serotonin and amplify serotonin neurotransmission beyond the effect of SSRI monotherapy. This antidepressant augmentation-like pharmacological effect in animals of 5-HTP SR was not associated with any apparent adverse events.
Evecxia’s pre-clinical data aligns with several clinical pilot studies conducted over the years with native 5-HTP. These studies reported that (i) adjunctive native 5-HTP can elevate brain extracellular serotonin beyond the first-line antidepressant effect, based on neuroendocrine biomarkers, and (ii) that adjunctive native 5-HTP can augment antidepressant efficacy in depressed patients, albeit often with bothersome CMax-related adverse events, and a requirement for multiple daily doses to maintain exposure.
Further, in a Phase 1 trial in healthy volunteers conducted by Evecxia, EVX-101 administered adjunctively to a first-line antidepressant demonstrated neuroendocrine biomarker evidence of extracellular serotonin elevation beyond the effect of the first-line antidepressant, which, everything equal, could equate augmented antidepressant efficacy in patients.
5-HTP

Serotonin

SSRI

Serotonin Transporter
Serotonin Receptor

Baseline
Serotonin is continuously released extracellularly into the synaptic cleft. Extracellular serotonin activates post-synaptic receptors that mediates serotonin neurotransmission. Pre-synaptic serotonin transporters work as a break on serotonin neurotransmission, by pumping extracellular serotonin back into the pre-synaptic neuron terminal.
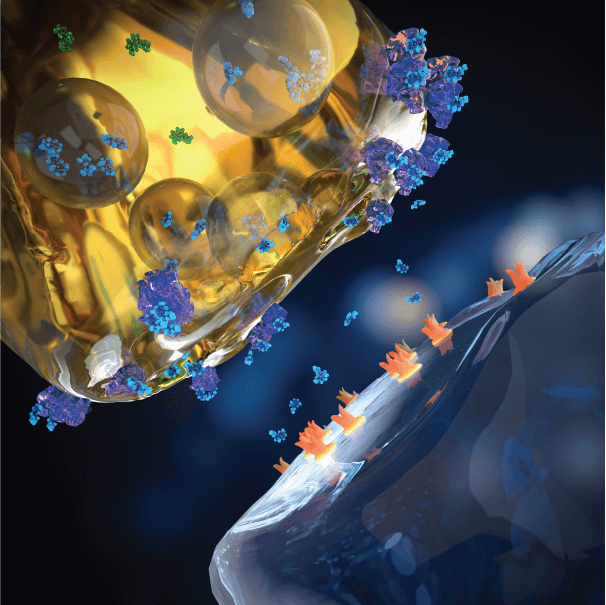
SSRI treated
Blocking the serotonin transporter with an SSRI is intended to elevate extracellular serotonin. SSRI treatment depends on baseline serotonin release, which limits how much and how reliably an SSRI (or SNRI) can enhance serotonin neurotranmission.
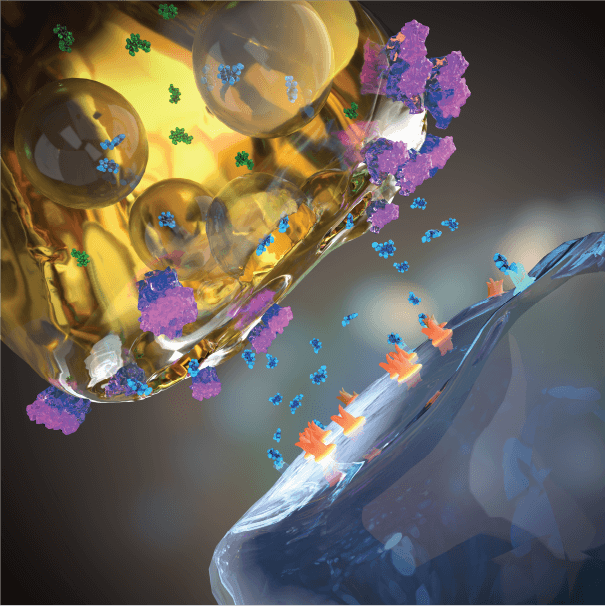
Adjunctive EVX-101 to SSRI treatment
Adjunctive EVX-101 amplifies neuronal serotonin production and release, thereby synergizing with SSRI (or SNRI) treatment in elevating extracellular serotonin and strengthening serotonin neurotransmission.
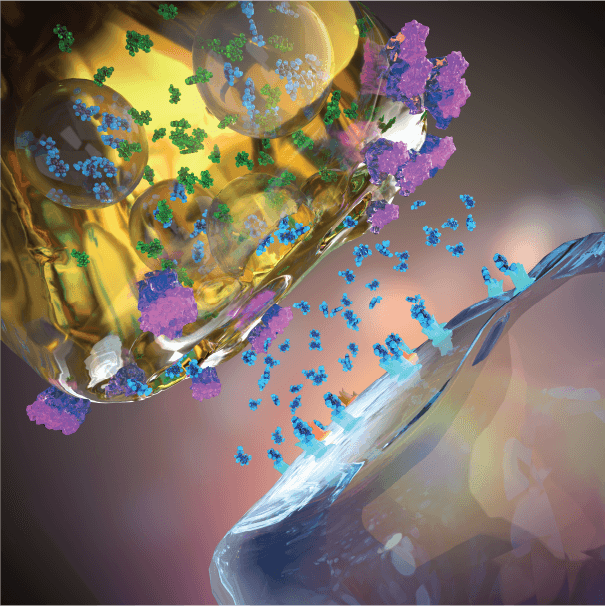
Technology Rationale
Overcoming 5-HTP’s Limitation As A Therapeutic
Native 5-HTP has 3 shortcomings as a therapeutic:
- Low bioavailability
- Poor fast in/fast out pharmacokinetics.
- Narrow absorption window restricted to the upper intestine.
Evecxia is employing proven drug delivery technologies and combinations with other compounds to remedy the short-comings of 5 HTP as a therapeutic, thereby for the first time enabling the therapeutic potential of serotonin synthesis amplification.
Overcoming 5-HTP’s Fast Absorption And Short Half-Life
Evecxia is developing drug delivery technologies that secures sustained 5-HTP plasma levels and consequently sustained brain serotonin amplification. These technologies optimize the therapeutic effect of 5-HTP and minimize adverse events.
Overcoming 5-HTP’s Narrow Absorption Window
In humans 5-HTP is substantially absorbed only by the upper intestine, and not by the colon. This narrow absorption window occludes using conventional drug delivery technologies to achieve sustained delivery of 5-HTP. Evecxia employs novel proprietary embodiments of validated technologies, to either deliver 5-HTP over a prolonged period to the upper intestine [EVX-101], or to deliver 5-HTP parenterally, bypassing the intestine [EVX-301]. These approaches enable for the first time to achieve sustained drug delivery of 5-HTP in humans.
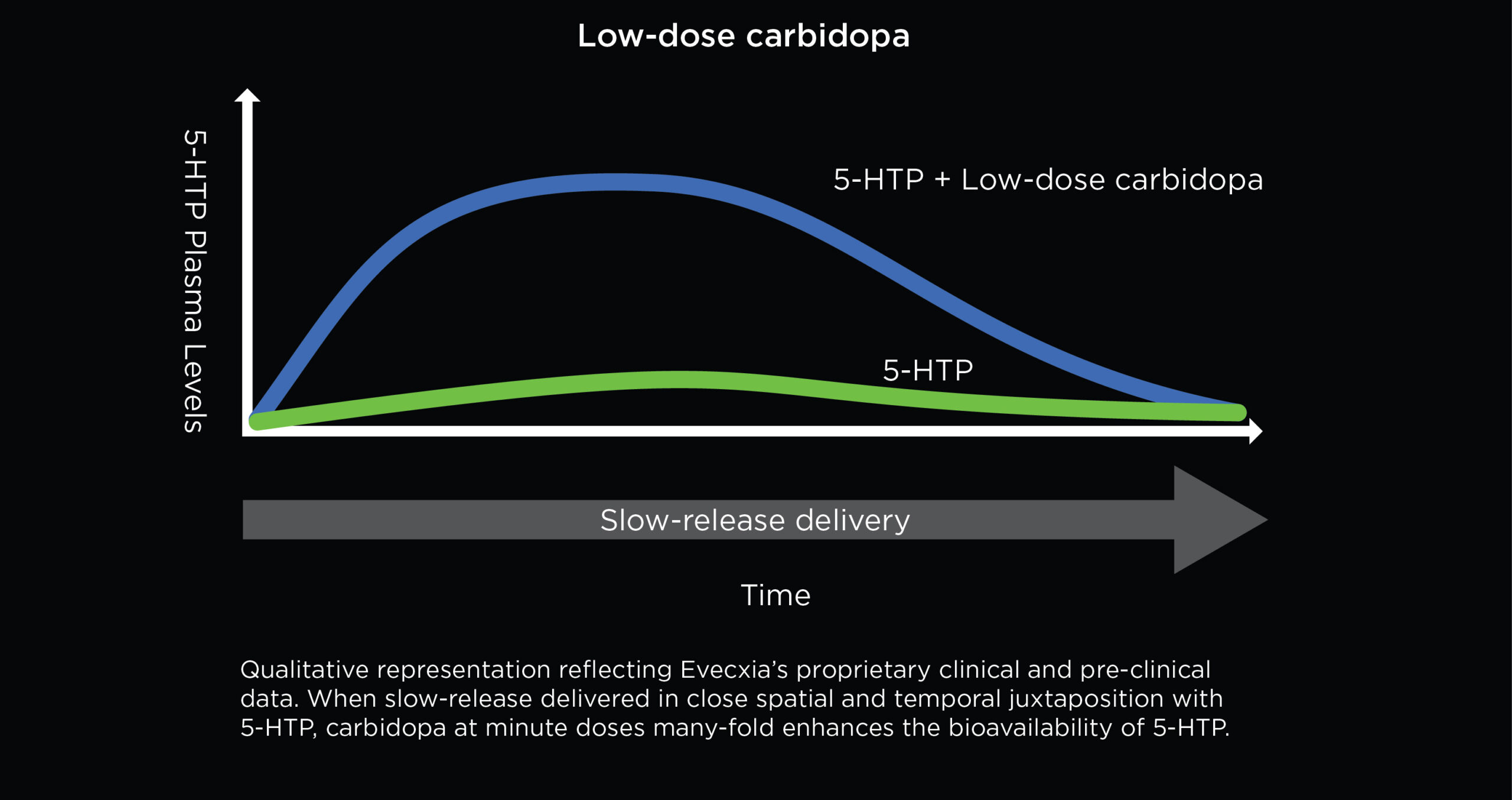
Overcoming 5-HTP’s Poor Absorption With Low-Dose Carbidopa
Carbidopa is a drug that inhibits aromatic L-amino acid decarboxylase, the enzyme that converts 5-HTP to serotonin, thus protecting 5-HTP against first-pass metabolism. Evecxia has discovered that extremely small doses of carbidopa can many-fold enhance 5-HTP’s absorption from the intestine. Carbidopa has previously been reported to enhance 5-HTP’s absorption, but in orders of magnitude higher doses. This unexpected pharmacological phenomenon requires that 5-HTP and carbidopa are delivered in close spatial and temporal juxtaposition to the site of 5-HTP absorption, the upper intestine. The low-dose carbidopa approach is used in EVX-101.
In another approach, 5-HTP is delivered via a constant IV infusion. This provides complete dose control, to optimize the rate of brain serotonin amplification, and to personalize the dose to the needs of the individual patient. This approach is used in EVX-301.